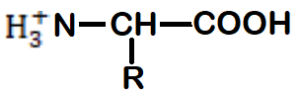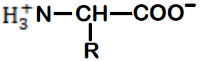9 BIOMOLECULES PRACTICE QUESTIONS
Elemental analysis on a plant tissue, Animal tissue or a microbial paste reveals:-
A) list of elements like C; H; O & several others
B) Respective content per unit mass of a living tissue
C) Both
D) Diversity of living organism in our Biosphere.
Elemental list could be _____ in _____terms of study on living tissues & earth’s crust:-
A) Same; absolute
B) Different; absolute
C) Different; same
D) Same; relative
With respect to other elements which element is relatively abundant in living organism than in earth’s crust:-
A) C & Ca
B) C & H
C) S & N
D) N & Ca
For the chemical composition analysis____ is used:-
A) 𝐶𝐻3𝐶𝑂𝑂𝐻
B) 𝐶𝐻3𝐶𝑂𝑂𝐻 – 𝐶𝑙
C) 𝐶𝑙3 – 𝐶𝐶𝑂𝑂𝐻
D) 𝐶𝑙3 – 𝐶𝑂𝑂𝐻
Filtrate obtained after grinding of living tissue is also known as:-
A) Slurry
B) Acid – soluble
C) Acid insoluble pool
D) All
Acid – insoluble pool is also known as:-
A) Slurry
B) Retentate
C) filtrate
D) All
Analytical techniques applied to the compound gives us an idea of:-
A) Probable structure of compounds
B) Molecular formula of compounds.
C) Both
D) None
All the carbon compounds that we get from the living tissue can be called:-
A) Biomolecules
B) Slurry
C) Retentate
D) All
If the tissue is fully burnt:-
A) All the carbon compounds are oxidised to gaseous forms (CO2 & water vapour).
B) Remaining’s are known as ash.
C) Ash contains inorganic elements & inorganic compounds.
D) All
Inorganic elements like sulphate and phosphates are present in
A) Ash of burnt tissue
B) Oxidised gaseous form
C) Both
D) None
α – Amino acids are organic compounds containing
A) Amino group and acidic group substituted on different carbon.
B) Keto – group & Hydrogen on different carbon.
C) Amino group & acidic group substituted on same carbon.
D) Keto – group & alcohol group substituted on same carbon.
How many substituted groups are present in an α – amino acid
A) 1
B) 2
C) 3
D) 4
The R – group in a proteinaceous amino acid could be
A) Hydrogen
B) Methyl group
C) Hydroxy methyl
D) Any of the above
The chemical and physical properties of amino acids are essentially of the
A) Amino group
B) Carboxyl group
C) The R – group
D) All of the above
If the R – group of amino acid is methyl
A) Glycine
B) Serine
C) Alanine
D) Any of the above
A hydrogen substituted carbon containing amino acid is :-
A) Glycine
B) Alanine
C) Both (A) & (B)
D) Serine
Number of Amino ; Carboxyl & the R – functional group determines:-
A) Acidic nature of Amino acid.
B) Basic nature of Amino acid
C) Neutral nature of Amino acid
D) Any of the above
Which of the following group of amino acid is aromatic in nature:-
A) tyrosine; phenylalanine
B) tyrosine; tryptophan glutamic acid
C) Glutamic acid; lysine; valine
D) none of the above
Which of the following is neutral in nature:-
A) Valine
B) Serine
C) Alanine
D) All
A particular property of amino acid is the ionizable nature of
A) -H
B) –NH2
C) CH3
D) All
Which of the following determines the particular property of amino acid is the Ionizable nature & structure of amino acid:-
A) –NH2 & -COOH
B) –COOH only
C) –NH2 only
D) none of the above
In different solution; of different ____ the ______ of amino acid changes.
A) pH; pH
B) pH; structure
C) Structure; Structure
D) structure; pH
Which of the following is a zwitterionic form.
(D) All of the above
Lipids are generally _____ insoluble:-
A) fat
B) water
C) Lipid
D) All
Lipids could be a ______ fatty acids or has a ______ group attached to an R – group.
A) Carboxyl; fatty acid
B) Fatty acid; simple
C) Carboxyl; simple
D) Simple; carboxyl
The R – group attached to the carboxyl group in a lipid could be a
A) –CH3
B) –C2H5
C) Higher number of –CH2
D) All of the above
Palmitic acid has _______ number of carbons including carboxyl carbon.
A) 16
B) 15
C) 14
D) 12
Arachidonic acid has _______ number of carbon atoms including the carboxyl
A) 16
B) 20
C) 21
D) 19
Fatty acids could be _______ ( with double bonds) or _______ ( without double bonds).
A) Saturated; Unsaturated
B) Unsaturated; Saturated
C) Saturated; Saturated
D) Unsaturated; Unsaturated
How many of the following is an esterified glycerol:-
Monoglyceride;
Diglyceride;
Triglyceride:
Muramic acid
Lignin;
Suberin
A) 4 B) 5 C) 6 D) 3
The oil have lower melting point
A) All fats B) triglycerides
C) Gingelly oil D) All
A phospholipid have
A) a phosphorous
B) a phosphorylated group
C) Both
D) None
The neural tissues have lipids with ______ structure
A) More complex B) Less complex
C) More simple D) simple
Carbon compounds in living organism having heterocyclic rings could be
A) Monoglyceride B) Adenine
C) Cytosine D) Both (B) & (C)
Adenine esterified with sugar is known as
A) Adenylic acid B) Adenosine
C) Adenotine D) None of the above
Nucleic acids like DNA & RNA consist of
A) Nucleotide & nucleoside
B) Nucleoside only
C) Nucleotide only
D) Nucleotide & phosphate groups.
Alkaloids; Flavonoids; Rubber; Essential oils; antibiotics; coloured pigments;scents; Gums spices
How many of the above are primary metabolites
A) 7 B) 9 C) 5 D) None
Few _______ metabolites have ecological importance’s:-
A) Primary & secondary B) Secondary & Primary
C) Only Primary D) Only Secondary
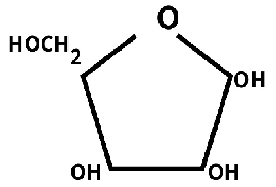
The diagram represent:-
A) Ribose B) Glucose C) Both D) None
CH3 – (CH2)14 – COOH
A) A glycerol molecule
B) A fatty acid
C) An amino acid
D) A carbohydrate
Which of the following is the compound represents the shown figure :-
A) A purine (Adenine) B) A pyrimidine (Uracil)
C) A purine (Uracil) D) A pyrimidine (Adenine)
Which of the following is a Nucleoside:-
A) Adenylic acid B) Uridine
C) Thymidylic acid D) All
How many of the following are nitrogen bases:-
i)  ii)
ii)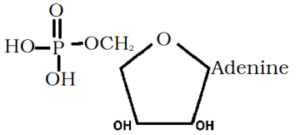
iii) Guanine
iv) Uracil
A) All four
B) Only three
C) Only two
D) Only one
They have molecular weight ranging from 18 to around 800 Da. The above written statement represents:-
A) About Biomacromolecules
B) One feature common to all those compounds found in the acid insoluble fraction.
C) Both
D) None
How many of the following statements are incorrect:-
i) Acid insoluble fraction has only four types of organic compounds.
ii) All the compound in acid insoluble fraction have molecular weight in range of 10,000 Da and above.
iii) Molecular weight less than one thousand Dalton are usually referred to as Micromolecules.
iv) Biomacromolecules are simply known as Biomolecules.
A) 1 B) 2 C) 3 D) 4
How many of the following statement is true regarding lipids in Biomacromolecules:-
i) Lipids are polymeric substances.
ii) Have molecular weight less than 10,000 Da.
iii) Molecular weight do not exceed 800 Da.
A) only i) & ii) B) only iii)
C) All i); ii) & iii) D) only ii) & iii)
Which of the following statement is not correct:-
A) After grinding cell membrane forms the vesicles.
B) Vesicles are water soluble.
C) Lipids are not strictly Biomacromolecules
D) None of the above
The acid soluble pool roughly represents______composition.
A) Cytoplasmic B) Nuclear C) Mitochondrial D) None
The macromolecules from the cytoplasm and organelles become the
A) Retentate
B) Slurry
C) Filtrate
D) All
Proteins are:-
A) Polypeptides
B) Linear chains of amino acid linked by peptide bonds
C) Polymer of amino acids
D) All of them.
A protein if a heteropolymer:-
A) It contains only one types of amino acids.
B) it contains different types of amino acids.
C) both
D) None
Which statement is incorrect:-
A) homopolymers have only one type of monomer repeating ‘n’ number of times
B) Dietary proteins are source of essential amino acids.
C) Amino acids could be essential or non – essential
D) essential amino acids are synthesized in our body.
What are functions of proteins:-
i) Carry out many functions in living organism
ii) Transporter of nutrients
iii) Fight infections
iv) Regulates in the form of hormones & enzymes
A) only two
B) only three
C) Only four
D) None
The most abundant enzyme in animal world is ___i)____ while in whole of the biosphere is___ii)____
A) (i) Collagen (ii) PEPcase
B) (i) RuBisCo (ii) PEPcase
C) (i) Collagen (ii) RuBisCO
D) None of them
Polysaccharide is the part of ____
A) In – soluble fraction
B) Insoluble pellet
C) Retentate
D) All
A polysaccharide contains
A) Different Monosacharides
B) Same type of monosaccharide
C) like cellulose
D) All of these
Cellulose and starch is a homopolymer of
A) Glucose
B) Fructose
C) Galactose
D) None
Which of the following statement is incorrect :-
A) starch is a a polysaccharide homopolymer.
B) Inulin is a polymer of fructose
C) In a polysaccharide chain, Right end is reducing while left end is non –reducing.
D) Starch forms helical secondary structures.
(I) Starch produces blue colour after binding with I2
(II) Cellulose cannot hold I2
A) Both are wrong
B) Both are correct
C) (I) is correct (II) is incorrect
D) (II) is correct (I) is incorrect
Paper made from plant pulp and cotton fibre is
A) Starch only
B) Cellulose
C) Complex polysaccharide
D) Both (B) & (C)
What are examples of homopolymers:-
A) N – acetyl galactosamine; Glucosamine
B) Amino acids; sugars
C) Chitin
D) None
Nucleic acids are:-
A) Polynucleosides
B) Polynucleotides
C) Both
D) None
A nucleotide has ____ chemical distinct compounds:-
A) Only one B) Two C) Three D) Four
A heterocyclic compound in Nucleic acid is :-
A) 𝑁2 – Base
B) Sugar
C) Fatty acid
D) All
Adenine and ______ are _______ purines
A) Cytosine; Substituted
B) Guanine; Substituted
C) Uracil; Substituted
D) Guanine; Unsubstituted
The sugar found in polynucleotides is either ribose (________) or ________
A) 2’ deoxyribose; monosaccharide B) Monosaccharide; 2’ deoxyribose
C) Disaccharide; 2’ deoxyribose D) Disaccharide; Monosaccheride
In a protein the left end represents:-
A) First amino acid & C – terminal
B) Last amino acid &N – terminal
C) First amino acid & N – terminal
D) Last amino acid & C – terminal
In a protein the right end represents
A) First amino acid & C – terminal
B) Last amino acid &N – terminal
C) First amino acid & N – terminal
D) Last amino acid & C – terminal
Which of the following statement is Untrue:-
A) A protein thread is folded in the form of a helix.
B) Only some portion of the protein thread are arranged in the form of a helix
C) In proteins only left handed helices are observed.
D) Both (B) & (C)
The long protein chain is also folded upon itself like a hollow woolen ball known as:-
A) Primary structure
B) Secondary Structure
C) Tertiary structure
D) None of the above
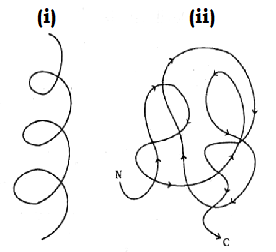
Identify the diagram given below
A) (i) Primary (ii) Secondary
B) (i) Secondary (ii) Tertiary
C) (i) Tertiary (iii) Primary
D) None of the above
Protein polypeptides or subunits arranged with respect to each other of a protein otherwise called the
A) Primary structure
B) Tertiary structure
C) Quaternary structure
D) Secondary structure
A adult human Hb (Haemoglobin) consists of ________ subunits.
A) 1
B) 2
C) 3
D) 4
________ subunits of α – type and ____ of β – type together constitute the human haemoglobin(Hb):-
A) 2; 4
B) 2; 2
C) 4; 2
D) 4; 4
In polypeptide amino acids are linked by
A) H – bond
B) Glycosidic Bond
C) Peptide bond
D) Peptide and H – bond both
Choose the correct statement about peptide bond
A) It is formed when carboxyl(-COOH) group of one amino acids react with carboxyl (-NH2) group of other amino acid.
B) It is formed when amino (-NH2) group of one amino acid react with carboxyl (-COOH) group of other amino acid.
C) It is formed when carboxyl group (-COOH) of one amino acid react with amino (-NH2) group of other amino acid.
D) It is formed when amino (-NH2) group of one amino acid react with amino (-NH2) group of other amino acid.
Peptide bond is formed by-
A) Elimination of water moiety i.e. rehydration
B) Addition of water moiety i.e. rehydration
C) Addition of water moiety i.e. dehydration
D) Elimination of water moiety i.e. dehydration
Polysaccharide is formed by linking of monosaccharide by-
A) H – bond B) S – bond C) Peptide Bond D) Glycoside bond
Dehydration is cause of formation of
A) Peptide bond B)Glycosidic bond
C) Both A & B D) None of these
Glycosidic bond is formed between monosaccharide while linking-
A) Carbon & Carbon B) Carboxyl & amino group
C) Carbon & Hydrogen D) Carbon & Oxygen
Match the Column- I & column – II
Bond Occurrence
(Column- I) Column – II
Peptide bond (i) Between Nitrogenous bases of nucleic acid
Glycosidic bond (ii) Between adjacent amino acid
Ester bond (iii) Between phosphate & hydroxyl group of sugar
H – Bond (iv) Between adjacent carbon of
monosaccharide
A) a – i, b – ii, c – iii, d – iv
B) a – ii , b – iv , c – i, d – iii
C) a – iii, b – iv, c – i, d – ii
D) a – ii, b – iv, c – iii, d – i
In nucleic acid phosphate links –
A) 3’ carbon of both sugar of succeeding sugar
B) 3’ carbon of one sugar & 5’ carbon of the other sugar of succeeding nucleotide
C) 5’ carbon of one sugar of succeeding sugar.
D) 5’ carbon of one sugar & 3’ carbon of other group of succeeding nucleotide.
What is / are number of ester bond & phosphodiester bond either side of nucleic acid respectively-
A) 1, 2 B) 1, 1 C) 2, 1 D) 2, 2
The famous Watson – crick model is related to–
A) Nucleic acid (DNA) B) Protein
C) Carbohydrate D) Enzymes
How many of following is / are correct with respect to Watson – crick model.
i) DNA exist as a double helix
ii) The strands of polynucleotides are antiparallel.
iii) Backbone is formed by sugar only.
iv) Nitrogen bases faces inside
v) A of one strand bound with U on other strand
A) 2
B)3
C) 4
D) All fives
Choose the correct nitrogen base pairing of DNA
A) A ≡ T
B) A = U
C) A = T
D) A ≡ U
Each step of ascent is represented by how many pairs of bases according to Watson – crick model.
A) 1
B) 2
C) Zero
D) None of these
At each of ascent, the strand turn ___
A) 63°
B) 36°
C) 34°
D) 3.4°
One full turn of helix strand of B –DNA involves how many nitrogen bases
A) 10
B) 20
C) 2
D) none of these
Choose correct statement regarding B –DNA
A) Pitch would be 36 A°
B) The rise per base pair would be 3.4A°
C) Pitch would be 3.4A°
D) The rise per base pair would be 36A°
Cytosine (C) bond with _____by _____ H –Bond.
A) Guanine (G); 2
B) Thymine; 2
C) Guanine (G); 3
D) Thymine; 3
What is ‘turn over’?
A) Biomolecules are never being changed into some other biomolecules and also made from some other biomolecules.
B) Biomolecules are constantly being changed into some other biomolecules but never made from
some other biomolecules.
C) Biomolecules are never being changed into some other biomolecules nor being made from
some other biomolecules.
D) Biomolecules are constantly being changed into some other biomolecules and also made from
some other biomolecules.
The breaking & making through chemical reaction which occur constantly in living organism are called
A) Metabolism B) Anabolism
C) Catabolism D) none of these
Amine are formed by-
A) removal of (-COOH) from amino acid
B) removal of (CO2) from amino acid
C) addition of (CO2) to amino acid
D) addition of (COOH) to amino acid
Metabolites are converted into each other in a series of linked reactions called________.
A) Catabolic pathway only
B) Anabolic pathway only
C) Metabolic pathway
D) None of these
Metabolic pathway are-
A) Linear only
B) Circular only
C) May be linear or circular
D) None of them
How many uncatalysed metabolic conversion is / are found in living system
A) 1
B) More than 1 but less than 100
C) Zero
D) Thousand
Metabolic pathway that lead to a more complex structure from a simples structure is / are
A) Anabolic pathway
B) Catabolic pathway
C) Both A & B
D) None of these
Choose the correct about catabolic pathway
i) Metabolic pathway that lead to simpler structure from a complex structure.
ii) Glucose becomes lactic acid in our skeletal muscles
iii) Acetic acid becomes cholesterol.
iv) Metabolic pathway that lead to more complex structure from a simpler structure.
A) i & iii
B) i & ii
C) iv & ii
D) iv & iii
Which of following expect to consume energy?
i) When glucose is degraded to lactic acid
ii) Assembly of protein from amino acid
iii) Anabolic pathway
iv) Catabolic pathway
A) i & iii
B) i & iv
C) ii & iii
D) ii & iv
How many of following is /are correct about glycolysis
i) Formation of glucose from lactic acid
ii) Occur in ten(10) metabolic step.
iii) Energy liberated during degradation is store in form of chemical bond.
iv) Formation of lactic acid from glucose
A) i, ii, iii
B)ii, iii, iv
C) i & ii
D) i & iv
Energy currency in living system is –
A) Adenosine triphosphate
B) Glucose
C) Protein
D) Enzyme
Bioenergetics deals with-
A) How do living organism derive their energy
B) How do living organism store energy & in what form.
C) How do living organism convert energy into work.
D) All of these
The blood concentration of glucose in normal healthy individuals is
A) Less than 2.4 mmol/L
B) More than 10 mmol/L
C) 4.2 mmol/L – 5.0 mmol/L
D) None of these
Living state is –
A) Equilibrium steady – state to be not to perform work.
B) Non – equilibrium steady – state to be not to perform work.
C) Equilibrium steady – state to be able to perform work.
D) Non – equilibrium steady – state to be able to perform work.
Living process is a constant effort to prevent falling into equilibrium. This is achieved by –
A) Energy output
B) energy input
C) Both of these
D) None of these
Enzymes are chemically –
A) Protein
B) Carbohydrate
C) Lipid
D) Nucleic acid
Ribozymes are chemically
A) Protein
B) Lipid
C) Carbohydrate
D) Nucleic acid
What is / are difference between inorganic catalyst and enzyme catalyst.
A) inorganic catalysts work efficiently at low temperature but enzyme of only thermophilic organism work efficiently at low temperature
B) Inorganic catalyst work efficiently at high temperature but enzyme get damaged at high temperature except of microbes that are live in sulphur springs
C) Inorganic catalyst are not efficient at high temperature but enzymes of all living organism work efficiently at high temperature.
D) None of these
Choose correct regarding “active site”
1) Substrate fits
2) Enzymes catalyst through active site show low rate
3) It forms by crevices or pocket made by primary protein only.
4) It form by crevices or pocket made by tertiary protein structure
A) 1, 2, 3
B) 1, 2, 4
C) 1, 3
D) 1, 4
Physical change refers to –
A) Change in shape without breaking bonds.
B) Change in state of matter
C) Ice 🡪 water 🡪 water vapour.
D) All of these
Chemical change differ from physical change in
A) Dissociation of bond
B) Formation of new bond
C) A & B bond
D) There is no difference in both
Hydrolysis of starch into glucose is :-
A) Inorganic chemical reaction
B) Organic chemical reaction
C) Physical changes
D) A & B both
Rate of physical or chemical process refer to –
A) Amount of reactant formed per unit time
B) Amount to product dissociate per unit time
C) Differential of time with respect to produce
D) Differential of product with respect to time
Choose the correct
A) Rate can be called velocity if the direction is not specific.
B) Rate of physical & chemical processes are not influenced by temperature
C) Catalysed reaction proceeds at rates vastly lower than that of uncatalyzed ones.
D) Catalysed reaction proceeds at rates vastly higher than that of uncatalyzed ones.
Choose the correct response
A) For every increase by 10°C, rate is double
B) Rate decrease by one – fourth by decrease in temperature by 10°
C) When enzymes catalysed reaction are observed the rate would be vastly lower than the same but uncatalyzed reaction.
D) None of these
Choose correct response with respect to given equation
Carbon dioxide + water ⇌ carbonic acid
A) Carbonic anhydrase is enzyme required for accelerated reaction.
B) In absence of enzyme, still this reaction is fast enough
C) 200 molecules of H2CO3 being per hour formed by enzyme accelerated reaction.
D) 600,000 molecules of H2CO3 being formed every second in absence of any enzyme.
Which of the following is correct chemical formula for pyruvic acid?
A) C2H3O4
B) C3H3O3
C) C3H4O3
D) C6H12O6
Match column – I and column – II
Column – I(Metabolic pathway) Column – II (Occurrence)
Formation of alcohol (i) Anaerobic condition of skeletal muscle
Formation of pyruvic acid (ii) Yeast
Formation of lactic acid (iii) Aerobic condition of normal human cell
A) A – I, B -iii, C -ii
B) A-iii, B -ii, C -I
C) A -ii, B -I, C -iii
D) None of these
Which of the following is correct about enzymes
A) It is 2 – D structure
B) Convert product into substrate
C) They have active site
D) All of these
Transition state structure is formed when –
A) Enzyme is free
B) Enzyme bound with product
C) ‘ES’ complex
D) Substrate structure do not change until product formed.
Which of following are unstable
A) Enzyme B) Product C) Reactant D) Intermediate structural states.
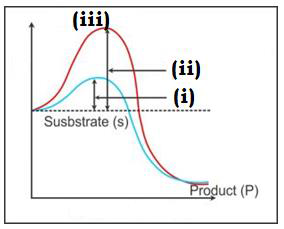
(i) (ii) (iii)
A) Activation energy without enzyme Transition state Activation energy with enzyme
B) Transition state Activation energy Activation energy with without enzyme enzyme
C) Activation energy with enzyme Activation energy Transition state
without enzyme
D) Activation energy without enzyme Activation with enzyme Transition state
Choose correct response
i) Y – axis represent potential energy
ii) X – axis represent substrate
iii) Y – axis represent progress of reaction
iv) X – axis represent state through transition state
A) i) & ii)
B) iii) & iv)
C) i) & iv)
D) ii) & iii
If ‘P’ (product) is at lower level than ‘s’ (substrate), the reaction is _______
A) Endothermic reaction
B) Exothermic reaction
C) Spontaneous reaction
D) A & C both
Which is correct way to represent enzyme action
A) E + S ES ⇌ EP ⇌ E + P
B) E + S ⇌ E + P
C) E + S ⇌ ES EP E + P
D) E + S ES EP ⇌ E +P
ES complex is _____ and dissociates into _______and__
A) Long lived; product; changed enzyme
B) Short lived; reactant, changed enzyme
C) Long lived, reactant, unchanged enzyme
D) Short lived, product, unchanged enzyme
Arrange in correct sequence of catalytic cycle of an enzyme action-
i) The active site of the enzyme, now in close proximity of the substrate breaks the chemical bonds of the
substrate and the new enzyme product complex is formed
ii) The substrate binds to the active site of enzyme, fitting into the active site
iii) The enzyme release the products of the reaction and the free enzyme is ready to bind to another molecule of the substrate
iv) The binding of the substrate induces the enzyme to alter its shape, fitting more tightly around the substrate.
A) i 🡪 ii 🡪 iii🡪 iv
B) i 🡪 iii 🡪 ii 🡪 iv
C) ii 🡪 iv 🡪 iii 🡪 I
D) ii 🡪 iv 🡪 i 🡪 iii
Which of the following can change enzyme activities?
A) All such activities that can alter the tertiary structure of the protein
B) Temperature pH
C) Substrate conditions
D) All of these
Enzyme activity decline-
A) Above the optimum value B) Below the optimum value
C) A & B both D) Enzyme activity never decline
Optimum pH refer to –
A) pH at which enzyme activity is lowest
B) pH at which enzyme activity is highest
C) pH at which enzyme activity started immediately
D) pH at which enzyme activity ended completely
choose response with respect to enzyme activities
i) low temperature destroy enzyme
ii) high temperature preserve enzyme in a temporarily inactive state
iii) optimum temperature is temperature at which enzyme activity is highest
iv) As temperature increase, enzyme activity increase until optimum and thereafter increase in temperature lead to decline in enzyme activities
v) As temperature increase enzyme activities is zero until optimum temperature & thereafter increase
in temperature lead to increase in enzyme activities
A) i, iii, iv
B) ii, v
C) i, iv, v
D) iii, iv
As pH increase, enzyme activity-
A) Constantly increase
B) Constantly decrease
C) No effect
D) Increase until optimum and decrease further pH
With increase in substrate concentration, the velocity of the enzymatic reaction –
A) Constantly increase
B) Rise at first until Vmax and further no rise
C) No effect
D) Decrease first until Vmax and increase further
After reaching Vmax, the enzymatic reaction does not exceed by any further rise in concentration of substrate because-
A) Enzymes molecules are fewer than substrate molecules
B) After saturation of those enzyme molecules these are no free enzyme molecules to bind with additional substrate molecules
C) A & B
D) After saturation of those enzyme molecules, enzyme get changed in it’s form.
When the binding chemical shut off enzyme activity, the process is called______ and the chemical is called
A) Inhibition; inhibitor B) Inhibition; cofactors
C) Exhibition, exhibitor D) None of these
What effect is observe on enzyme activity due to inhibitor
A) It fasten enzyme kinetics
B) It decline enzyme kinetics
C) It shut off enzyme kinetics
D) No effect on enzyme kinetics
Inhibition of succinic dehydrogenase by malonate is due to
A) Malonate closely resembles with substrate succinate in structure
B) Malonate is competitive inhibitor
C) It binds with active site of succinic dehydrogenase in place of substrate
D) All of these
Competitive inhibitors are often used in the control of –
A) Viral pathogen
B) Bacterial pathogen
C) Both A & B
D) None of these
Enzyme are divided into how many classes-
A) 2
B) 4
C) 6
D) 8
Each classes of enzyme were further classification into______ subclass and named by ___ digit
A) 13; 4 – 13
B) 4 – 13; 13
C) 4 – 13; 4
D) 4; 4 – 13
S reduced + S’ oxidised ⟶ S oxidised + S’ reduced
A) Oxidoreductase
B) Dehydrogenase
C) Transferase
D) A & B both
Enzyme catalysing a transfer of a group i.e. hydrogen between pair of substrate S and S’ is-
A) Transferase
B) Oxidoreductase
C) Lyases
D) Ligases
Transferase enzyme catalyse a transfer of G between pair substrate S & S’. G is other than –
A) Oxygen
B) Amino
C) Hydrogen
D) Carbon
Hydrolases catalyse –
i) Hydrolysis of ester, ether, peptide, glycosidic,
ii) C – C breakdown
iii) C – halide breakdown
iv) P – N breakdown
A) (i) only
B) (i) & (ii) only
C) (iii) & (iv) only D)
D) i, ii, iii & iv
Lysase catalyse _ _ _ _ _ _ of groups from substrates by mechanism other than hydrolysis leaving _ _ _ bond.
Addition ; double B. Removal ; double
Addition ; single D. Removal ; triple
Isomerases catalyse inter-conversion of:
Optical isomer B. Geometrical isomer
Position isomer D. All of these
Linking of two compound is achived by- [
A) Lyases
B) Transferase
C) Ligases
D) Hydrolase
Ligase catalyse-
A) Joining of C-O
B) Oxidation – reduction of substrate
C) Hydrolysis of C-C
D) Conversion of optical isomer
Cofactors are:-
A) Proteinous part of enzyme
B) Non-proteinous part of enzyme
C) Bound to substrate
D) Bound to enzyme to make enzyme catalytically retard
How many kind of cofactors may be identified–
A) 1
B) 2
C) 3
D) Zero
Cofactors are________and apoenzyme are _ _ _ _ part of enzyme.
A) Protein; protein
B) Non-protein; non-protein
C) Protein; non-protein
D) Non-protein; protein
Prosthetic group are _ _ _ _ _ and are distinguished from other cofactors in that they are _ _ _ _ _ bound to apoenzyme.
A) Organic compound; tightly
B) Organic compound; loosely
C) Inorganic compound; loosely
D) Inorganic compound; tightly
Which of following is/are correct?
(i) Haem is prosthetic group.
(ii) Haem is apoenzyme.
(iii) Haem is not part of active site of peroxidase.
(iv) Haem catalyse the formation of hydrogen peroxide from water & oxygen.
(v) Haem is part of active site of peroxidase.
(vi) Haem catalyse the breakdown of hydrogen peroxide into water & oxygen.
A) i , iii , vi
B) ii , iv , v
C) i , v , vi
D) ii , v , vi
NAD & NADP contain-
A) Vitamin niacin
B) Vitamin C
C) Vitamin D
D) Vitamin K
Full form of NAD is:-
A) Nicotinamide adenine nucleotide
B) Nicotinamide adenine dinucleoside
C) Nicotinamide adenine dinucleotide
D) Nicotinamide adenine nucleoside
Choose correct response from following with respect to carboxypeptidase.
A) Zinc are found as apoenzyme
B) It is proteolytic enzyme
C) Cofactor from covalent bond with side chain at active site
D) Between cofactor and substrate ionic bond is formed
How many coordination found in activity of carboxypeptidase?
A) Only one ; between cofactor and side chain at active site
B) Two between cofactor and side chain at active site and at to many ; same time form one or more bond with substrate.
C) Zero
D) Only one ; between cofactor & substrate
Find mismatch.
Column-I Column-II
(a) Carboxypeptidase (i) Zinc
(b) NADP (ii) Niacin
(c) Haem (iii) Peroxidase
(d) NAD (iv) Zinc
When cofactor is removed from enzyme ; what effect is observed.
A) Catalytic activity lost
B) Catalytic activity enhance
C) Catalytic activity fix at optimum
D) None of these
The two functional groups characteristic of sugars are
(a) Hydroxyl and methyl
(b) Carbonyl and methyl
(c) Carbonyl and hydroxyl
(d) Carbonyl and phosphate
Which of the following are not polymeric?
(a) Proteins
(b) Polysaccharides
(c) Lipids
(d) Nucleic acids
Which one of the following statements is correct with reference to enzymes?
(a) Holoenzyme = Apoenzyme + Coenzyme
(b) Coenzyme = Apoenzyme + Holoenzyme
(c) Holoenzyme = Coenzyme + Co-factor
(d) Apoenzyme = Holoenzyme + Coenzyme
A typical fat molecule is made up of
(a) three glycerol molecules and one fatty acid molecule.
(b) one glycerol and three fatty acid molecules.
(c) one glycerol and one fatty acid molecule.
(d) three glycerol and three fatty acid molecules.
Which one of the following statements is incorrect?
(a) Sucrose is a disaccharide.
(b) Cellulose is a polysaccharide.
(c) Uracil is a pyrimidine.
(d) Glycine is a sulphur containing amino acid.
The amino acid Tryptophan is the precursor for the synthesis of
(a) melatonin and serotonin.
(b) thyroxine and triiodothyronine.
(c) estrogen and progesterone.
(d) cortisol and cortisone.
Which of the following biomolecules does have phosphodiester bond?
(a) Monosaccharides in a polysaccharide.
(b) Amino acids in a polypeptide.
(c) Nucleic acids in a nucleotide.
(d) Fatty acids in a diglyceride.
Which one of the following statements is incorrect?
(a) In competitive inhibition, the inhibitor molecule is not chemically changed by the enzyme.
(b) The competitive inhibitor does not affect the rate of breakdown of the enzyme-substrate complex.
(c) The presence of the competitive inhibitor decreases the Km of the enzyme for the substrate.
(d) A competitive inhibitor reacts reversibly with the enzyme to form an enzyme- inhibitor complex.
Which one of the following is a non-reducing carbohydrate?
(a) Maltose
(b) Sucrose
(c) Lactose
(d) Ribose 5 – phosphate
Select the statement which is not correct with respect to enzyme action.
(a) Substrate binds with enzyme at its active site.
(b) Addition of lot of succinate does not reverse the inhibition of succinic dehydrogenase by malonate.
(c) A non-competitive inhibitor binds the enzyme at a site distinct from that which binds the substrate.
(d) Malonate is a competitive inhibitor of succinic dehydrogenase.
Which of the following glucose transporters is insulin-dependent?
(1) GLUT I
(2) GLUT II
(3) GLUT III
(4) GLUT IV
Concanavalin A is :
(1) an alkaloid
(2) an essential oil
(3) a lectin
(4) a pigment
Consider the following statements :
(A) Coenzyme or metal ion that is tightly bound to enzyme protein is called prosthetic group.
(B) A complete catalytic active enzyme with its bound prosthetic group is called apoenzyme.
Select the correct option.
(1) Both (A) and (B) are true.
(2) (A) is true and (B) is false.
(3) Both (A) and (B) are false.
(4) (A) is false and (B) is true.
Which of the following organic compounds is the main constituent of Lecithin?
(1) Arachidonic acid
(2) Phospholipid
(3) Cholesterol
(4) Phosphoprotein
Prosthetic groups differ from co-enzymes in that :-
(1) they require metal ions for their activity.
(2) they (prosthetic groups) are tightly bound to apoenzymes.
(3) their association with apoenzymes is transient.
(4) they can serve as co-factors in a number of enzyme-catalyzed reactions.
Identify the statement which is incorrect.
(1) Sulphur is an integral part of cysteine.
(2) Glycine is an example of lipids.
(3) Lecithin contains phosphorus atom in its structure.
(4) Tyrosine possesses aromatic ring in its structure.
Identify the substances having glycosidic bond and peptide bond, respectively in their structure:
1) Inulin, insulin
2) Chitin, cholesterol
3) Glycerol, trypsin
4) Cellulose, lecithin
Match the following:
(a) Inhibitor of catalytic activity (i) Ricin
(b) Possess peptide bonds (ii) Malonate
(c) Cell wall material in fungi (iii) Chitin
(d) Secondary metabolite (iv) Collagen
Choose the correct option from the following:
(a) (b) (c) (d)
1) (ii) (iii) (i) (iv)
2) (ii) (iv) (iii) (i)
3) (iii) (i) (iv) (ii)
4) (iii) (iv) (i) (ii)
Which of the following statements is correct?
1) Adenine does not pair with thymine
2) Adenine pairs with thymine through two H-bonds
3) Adenine pairs with thymine through one H-bond
4) Adenine pairs with thymine through three H-bonds
Which one of the following is the most abundant protein in the animals?
1) Insulin
2) Haemoglobin
3) Collagen
4) Lectin
Identify the basic amino acid from the following
1) Valine
2) Tyrosine
3) Glutamic Acid
4) Lysine
Which of the following are not secondary metabolites in plants ?
1)Amino acids, glucose
2) Vinblastin, curcumin
3) Rubber, gums
4) Morphine, codeine
Match List – I with List – II
List – I |
List – II |
||
| a) | Protein | i) | C = C double bonds |
| b) | Unsaturated fatty acid | ii) | Phosphodiester bonds |
| c) | Nucleic acid | iii) | Glycosidic bonds |
| d) | Polysaccharide | iv) | Peptide bonds |
a b c d
1) i iv iii ii
2) ii i iv iii
3) iv iii i ii
4) iv i ii iii
Identify the incorrect pair
1) Toxin – Abrin
2) Lectins – Concadnavalin A
3) Drugs – Ricin
4) Alkaloids – Codeine
Following are the statements with reference to ‘lipids’
a) Lipids having only single bonds are called unsaturated fatty acids
b) Lecithin is a phospholipid
c) Trihydroxy propane is glycerol.
d) Palmitic acid has 20 carbon atoms including carboxyl carbon.
e) Arachidonic acid has 16 carbon atoms.
Choose the correct answer from the options given below.
1) c and d only
2) b and c only
3) b and e only
4) a and b only
Read the following statements on lipids and find out correct set of statements:
a) Lecithin found in the plasma membrane is a glycolipid
b) Saturated fatty acids possess one or more c = c bonds
c) Gingelly oil has lower melting point, hence remains as oil in winter
d) Lipids are generally insoluble in water but soluble in some organic solvents
e) When fatty acid is esterified with glycerol, monoglycerides are formed
Choose the correct answer from the options given below:
1) a, b and c only
2) a, d and e only
3) c, d and e only
4) a, b and d only
A dehydration reaction links two glucose molecules to produce maltose. If the formula for glucose is then what is the formula for maltose?
1) 2) 3) 4)
Match List-I with List-II
List-I List-II
(Biological Molecules) (Biological functions)
(a) Glycogen (i) Hormone
(b) Globulin (ii) Biocatalyst
(c) Steroids (iii) Antibody
(d) Thrombin (iv) Storage product
Choose the correct answer from the options given below:
1) (a) –(iii), (b) –(ii), (c) – (iv), (d) – (i)
2) (a) –(iv), (b) – (ii), (c) – (i), (d) – (iii)
3) (a) –(ii), (b) – (iv), (c) – (iii), (d) – (i)
4) (a) –(iv), (b) – (iii), (c) – (i), (d) – (ii)